MORE QUESTIONS SOLVED
I. Very Short Answer Type Questions
Question 1. Give the relation between wavelength and momentum of moving microscopic particle. What is the relation known as?
Answer:
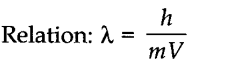
The relation is known as de Broglie’s relationship.
Question 1. Give the relation between wavelength and momentum of moving microscopic particle. What is the relation known as?
Answer:
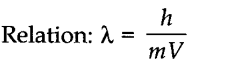
The relation is known as de Broglie’s relationship.
Question 2. Write the electronic configuration and number of unpaired electrons in Fe2+ion.
Answer: Fe (Z = 26) : [Ar]18 3d64s2
Fe2+ion : [Ar]18 3d6
No. of unpaired electrons = 4
Answer: Fe (Z = 26) : [Ar]18 3d64s2
Fe2+ion : [Ar]18 3d6
No. of unpaired electrons = 4
Question 3. What are degenerate orbitals ?
Answer: Orbitals having same energy belonging to the same subshell.
Answer: Orbitals having same energy belonging to the same subshell.
Question 4. What is the most important application of de Broglie concept?
Answer: In the construction of electron microscope used for the measurement of objects of very small size.
Answer: In the construction of electron microscope used for the measurement of objects of very small size.
Question 5. Which one Fe3+, Fe2+is more paramagnetic and why?
Answer: As Fe3+ contains 5 impaired electrons while Fe2+ contains only 4 unpaired electrons. Fe3+ is more paramagnetic.
Answer: As Fe3+ contains 5 impaired electrons while Fe2+ contains only 4 unpaired electrons. Fe3+ is more paramagnetic.
Question 6. Which element does not have any neutron?
Answer: Hydrogen.
Answer: Hydrogen.
Question 7. What is value of Planck’s constant in S.I. units?
Answer: 6.62 x 1034 Js.
Answer: 6.62 x 1034 Js.
Question 8. Arrange X-rays, cosmic rays and radio waves according to frequency.
Answer: Cosmic rays > X-rays > radio waves.
Answer: Cosmic rays > X-rays > radio waves.
Question 9. Which series of lines of the hydrogen spectrum lie in the visible region?
Answer: Balmer series.
Answer: Balmer series.
Question 10. What is the difference between ground state and excited state?
Answer: Ground state means the lowest energy state. When the electrons absorb energy and jump to outer orbits, this state is called excited state.
Answer: Ground state means the lowest energy state. When the electrons absorb energy and jump to outer orbits, this state is called excited state.
Question 11. What is common between dxy and dx2-y 2 orbitals?
Answer: Both have identical shape, consisting of four lobes.
Answer: Both have identical shape, consisting of four lobes.
Question 12. If n is equal to 3, what are the values of quantum numbers l and m?
Answer: I = 0,1, 2
m =- 2, — 1, 0, + 1, + 2 and S = +1/2 and-1/2
for each value of m.
Answer: I = 0,1, 2
m =- 2, — 1, 0, + 1, + 2 and S = +1/2 and-1/2
for each value of m.
Question 13.
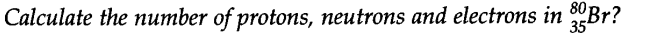 Answer: Z = 35
Answer: Z = 35
A = 80
Atomic no. = 35 No. of protons = 35
No. of protons No. of electrons No. of neutrons
= 80 – 35 = 45
A = 80
Atomic no. = 35 No. of protons = 35
No. of protons No. of electrons No. of neutrons
= 80 – 35 = 45
Question 14. An electron beam after hitting a neutral crystal produces a diffraction pattern? What do you conclude?
Answer: Electron has wave nature.
Answer: Electron has wave nature.
Question 15. An electron beam on hitting a ZnS screen produces a scientillation on it. What do you conclude?
Answer: Electron has particle nature.
Answer: Electron has particle nature.
Question 16. Discuss the similarities and differences between a 1s and a 2s orbital.
Answer: Similarities:
Answer: Similarities:
- Both have spherical shape.
- Both have same angular momentum.
Differences:
- 1s has no node while 2s has one node.
- Energy of 2s is greater than that of 1s.
Question 17. What mil be the order of energy levels 3s, 3p and 3d in case of H-atom?
Answer: All have equal energy.
Answer: All have equal energy.
Question 18. How many unpaired electrons are present in Pd (Z = 46) ?
Answer: The electronic configuration of the element palladium (Z = 46) is [Kr]36 4d10 5S°.
This means that it has no impaired electron.
Answer: The electronic configuration of the element palladium (Z = 46) is [Kr]36 4d10 5S°.
This means that it has no impaired electron.
Question 19. Distinguish between a photon and quantum.
Answer: A quantum is a bundle of energy of a definite magnitude (E = hv) and it may be from any source. However, a photon is quantum of energy associated with light only.
Answer: A quantum is a bundle of energy of a definite magnitude (E = hv) and it may be from any source. However, a photon is quantum of energy associated with light only.
Question 20. What type of metals are used in photoelectric cells? Give one example.
Answer: The metals with low ionisation enthalpies are used in photoelectric cells. Caesium (Cs), an alkali metal belonging to group 1 is the most commonly used metal.
Answer: The metals with low ionisation enthalpies are used in photoelectric cells. Caesium (Cs), an alkali metal belonging to group 1 is the most commonly used metal.
Question 21. When is the energy of electron regarded as zero?
Answer: The energy of the electron is regarded as zero when it is at infinite distance from the nucleus.
At that point force of attraction between the electron and the nucleus is almost nil. Therefore, its energy is regarded as zero.
Answer: The energy of the electron is regarded as zero when it is at infinite distance from the nucleus.
At that point force of attraction between the electron and the nucleus is almost nil. Therefore, its energy is regarded as zero.
Question 22. What is difference between the notations l and L?
Answer: ‘l’ signifies the secondary quantum number.
‘L’ signifies second energy level (n = 2).
Answer: ‘l’ signifies the secondary quantum number.
‘L’ signifies second energy level (n = 2).
II. Short Answer Type Questions
Question 1. The uncertainty in the position of a moving bullet of mass 10 g is 10-5 m. Calculate the uncertainty in its velocity?
Answer: According to uncertainty principle,
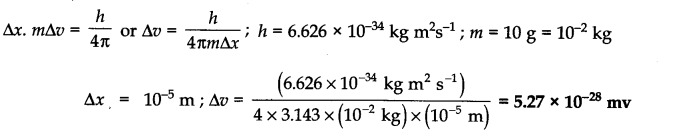
Question 1. The uncertainty in the position of a moving bullet of mass 10 g is 10-5 m. Calculate the uncertainty in its velocity?
Answer: According to uncertainty principle,
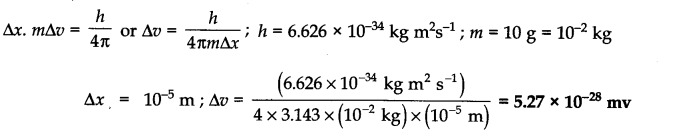
Question 2. The uncertainty in the position and velocity of a particle are 10-10m and 5.27 x 10-24 ms-1 respectively. Calculate the mass of the particle. (Haryana Board 2000)
Answer: According to uncertainty principle,
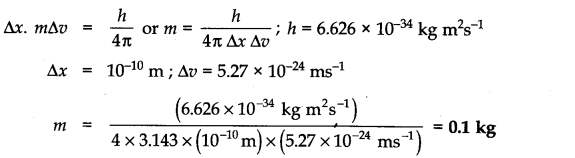
Answer: According to uncertainty principle,
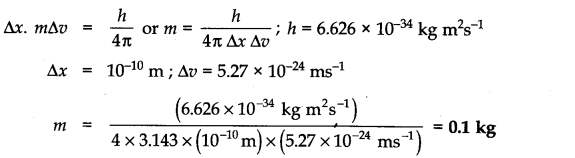
Question 3. With what velocity must an electron travel so that its momentum is equal to that of a photon of wavelength = 5200 A?
Answer:
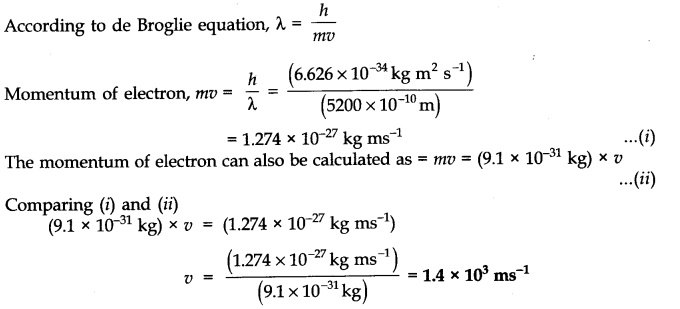
Answer:
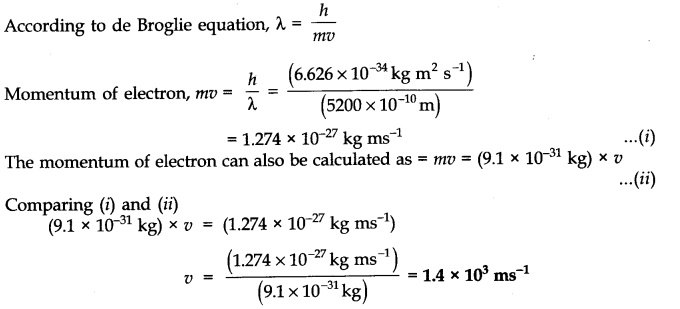
Question 4. Using Aufbau principle, write the ground state electronic configuration of following atoms.
(i)Boron (Z = 5) (ii) Neon (Z = 10), (iii) Aluminium (Z = 13) (iv) Chlorine (Z = 17), (v) Calcium (Z = 20) (vi) Rubidium (Z = 37)
Answer: (i)Boron (Z = 5) ; 1s2 2s2 1p1
(ii)Neon (Z = 10) ; 1s2 2s2 2p6
(iii)Aluminium (Z = 13) ; 1s2 2s2 2p6 3s2 3p1
(iv)Chlorine(Z = 17) ; 1s2 2s2 2p6 3s2 3p5
(v)Calcium (Z = 20) ; 1s2 2s2 2p6 3s2 3p6 4s2
(vi)Rubidium (Z = 37) ; 1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s1.
(i)Boron (Z = 5) (ii) Neon (Z = 10), (iii) Aluminium (Z = 13) (iv) Chlorine (Z = 17), (v) Calcium (Z = 20) (vi) Rubidium (Z = 37)
Answer: (i)Boron (Z = 5) ; 1s2 2s2 1p1
(ii)Neon (Z = 10) ; 1s2 2s2 2p6
(iii)Aluminium (Z = 13) ; 1s2 2s2 2p6 3s2 3p1
(iv)Chlorine(Z = 17) ; 1s2 2s2 2p6 3s2 3p5
(v)Calcium (Z = 20) ; 1s2 2s2 2p6 3s2 3p6 4s2
(vi)Rubidium (Z = 37) ; 1s2 2s2 2p6 3s2 3p6 3d104s2 4p6 5s1.
Question 5. Calculate the de Broglie wavelength of an electron moving with 1% of the speed of light?
Answer:

Answer:

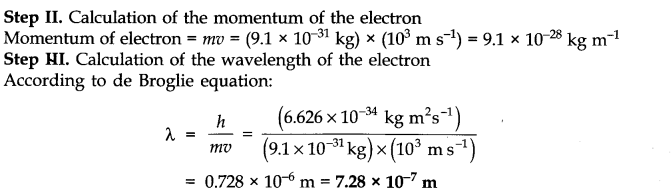
Question 6. The kinetic energy of an electron is 4.55 x 10-25 J. The mass of electron 9.1 x 10-1kg. Calculate velocity, momentum and the wavelength of the electron?(Haryana Board, 2004, AII CBSE 2000)
Answer:
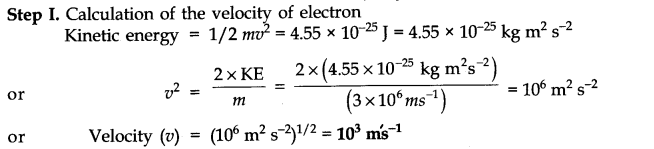
Answer:
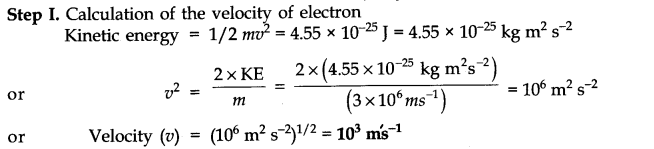

Question 7. What is the wavelength for the electron accelerated by 1.0 x 104 volts?
Answer:
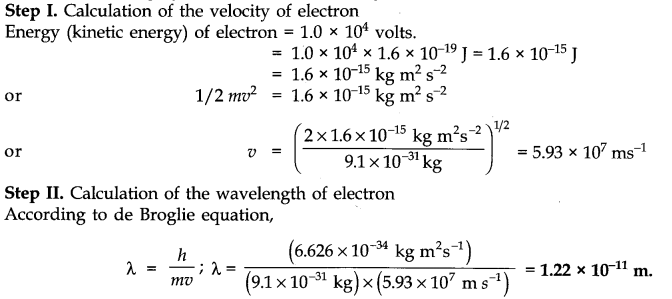
Answer:

Question 8. In a hydrogen atom, the energy of an electron in first Bohr’s orbit is 13.12 x 105 J mol-1. What is the energy required for its excitation to Bohr’s second orbit?
Answer: The expression for the energy of electron of hydrogen is:
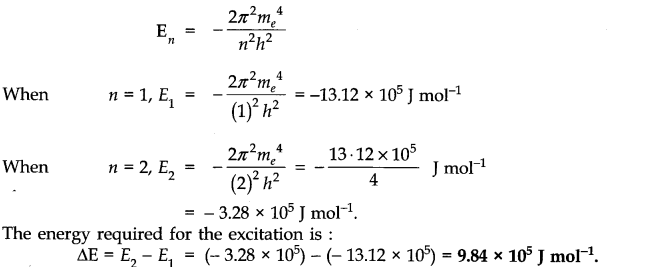
Answer: The expression for the energy of electron of hydrogen is:
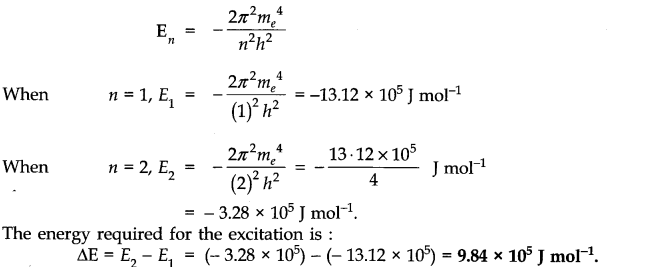
Question 9. What are the two longest wavelength lines (in manometers) in the Lyman series of hydrogen spectrum?
Answer: According to Rydberg-Balmer equation.
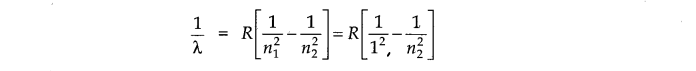
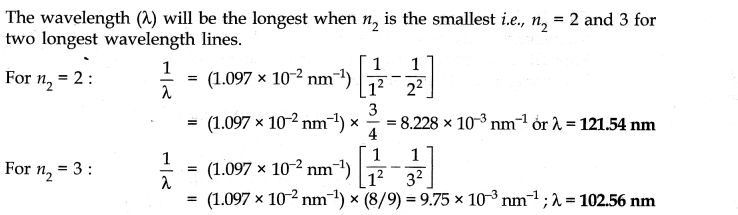
Answer: According to Rydberg-Balmer equation.
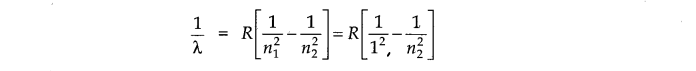
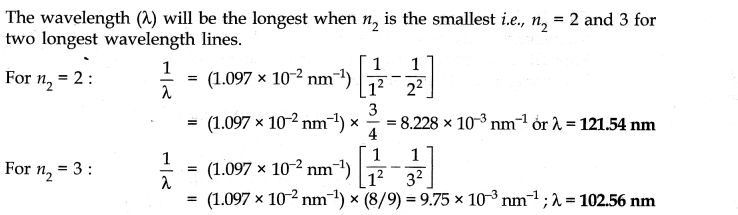
III. Long Answer Type Questions
Question 1. (a) What is the limitations of Rutherford model of atoms?
(b) How has Bohr’s theory helped in calculating the energy of hydrogen electron in different energy levels?
Answer: (a) Limitations of Rutherford Model:
(i) When a body is moving in an orbit, it achieves acceleration (even if body is moving with constant speed in an orbit, it achieves acceleration due to change in direction). So an electron moving around nucleus in an orbit is under acceleration. However, according to radiation theory of Maxwell, the charged particles when accelerated must emit energy as electromagnetic radiations. This means that the revolving electron must also lose energy continuously in the form of electromagnetic radiation. The loss of energy in revolution of the electron around the nucleus must bring it closer to the nucleus and the electron must ultimately fall into the nucleus by the spiral path. This means that the atom must collapse. But we all know that atom is quite stable in nature.
(ii) Rutherford’s model could not explain the existence of different spectral lines in the hydrogen spectrum.
(b) Based upon the postulates of Bohr’s theory, it is possible to calculate the energy of the hydrogen electron and also one electron species. (He+, Li2+ etc.) The mathematical expression for the energy in the nth orbit is
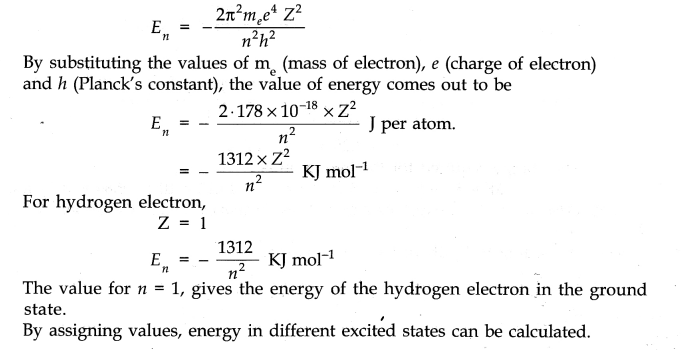
Question 1. (a) What is the limitations of Rutherford model of atoms?
(b) How has Bohr’s theory helped in calculating the energy of hydrogen electron in different energy levels?
Answer: (a) Limitations of Rutherford Model:
(i) When a body is moving in an orbit, it achieves acceleration (even if body is moving with constant speed in an orbit, it achieves acceleration due to change in direction). So an electron moving around nucleus in an orbit is under acceleration. However, according to radiation theory of Maxwell, the charged particles when accelerated must emit energy as electromagnetic radiations. This means that the revolving electron must also lose energy continuously in the form of electromagnetic radiation. The loss of energy in revolution of the electron around the nucleus must bring it closer to the nucleus and the electron must ultimately fall into the nucleus by the spiral path. This means that the atom must collapse. But we all know that atom is quite stable in nature.
(ii) Rutherford’s model could not explain the existence of different spectral lines in the hydrogen spectrum.
(b) Based upon the postulates of Bohr’s theory, it is possible to calculate the energy of the hydrogen electron and also one electron species. (He+, Li2+ etc.) The mathematical expression for the energy in the nth orbit is
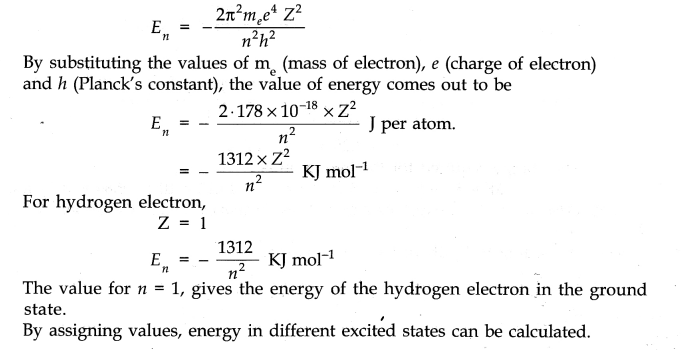
Question 2. Define atomic number, mass number and neutron. How are the three related to each other?
Answer: Atomic Number (Z): The atomic number of an element is equal to the number of protons present inside the nucleus of its atoms.
Since, an isolated atom has no net charge on it, in neutral atoms, the total number of electrons is equal to its atomic number.
Atomic number (Z) = Number of protons in the nucleus of an atom = Number of electrons in the neutral atomsMass Number (A): The sum of the number of neutrons and protons in the nucleus of an atom is called its mass number. Mass number is denoted by A. Thus, for an atom, Mass number (A) = Number of protons (p) + Number of neutrons (n)
A = p + n
Neutron: It is neutral particle. It is present in the nucleus of an atom. Expect hydrogen (which contains only one electron and one proton but no neutron), the atoms of all other elements including isotopes of hydrogen contain all the three fundamental particles called neutron, proton and electron.
The relation between mass number, Atomic no. and no. of neutrons is given by the equation:
Where A = Mass number
Z = Atomic number n = Number of neutrons in the nucleus.
Answer: Atomic Number (Z): The atomic number of an element is equal to the number of protons present inside the nucleus of its atoms.
Since, an isolated atom has no net charge on it, in neutral atoms, the total number of electrons is equal to its atomic number.
Atomic number (Z) = Number of protons in the nucleus of an atom = Number of electrons in the neutral atomsMass Number (A): The sum of the number of neutrons and protons in the nucleus of an atom is called its mass number. Mass number is denoted by A. Thus, for an atom, Mass number (A) = Number of protons (p) + Number of neutrons (n)
A = p + n
Neutron: It is neutral particle. It is present in the nucleus of an atom. Expect hydrogen (which contains only one electron and one proton but no neutron), the atoms of all other elements including isotopes of hydrogen contain all the three fundamental particles called neutron, proton and electron.
The relation between mass number, Atomic no. and no. of neutrons is given by the equation:
Where A = Mass number
Z = Atomic number n = Number of neutrons in the nucleus.
Question 3. What were the weaknesses or limitations of Bohr’s model of atoms ? Briefly describe the quantum mechanical model of atom.
Answer: Limitations of Bohr’s model of an atom:
Answer: Limitations of Bohr’s model of an atom:
- It could not explain spectrum of multi-electron atoms.
- It could not explain Zeeman and Stark effects.
- It could not explain shape of molecules.
- It was not in accordance with Heisenberg’s uncertainty principle.
Quantum Mechanical Model: It was developed on the basis of Heisenberg’s uncertainty principle and dual behaviour of matter.
Main features of this model are given below :
- The energy of electrons in an atom is quantized i.e. can only have certain values.
- The existence of quantized electronic energy levels is a direct result of the wave like properties of electrons.
- Both, the exact position and velocity of an electron in an atom cannot be determined simultaneously.
- The orbitals are filled in increasing order of energy. All the information about the electron in an atom is stored in orbital wave function Ψ.
- From the value of Ψ2 at different points within atom, it is possible to predict the region around the nucleus where electron most probably will be found.
Question 4. State and explain the following:
(i) Aufbau principle
(ii) Pauli exclusion principle.
(iii) Hund’s rule of maximum multiplicity.
Answer: (i) Aufbau Principle: In the ground state of the atoms, the orbitals are filled in the order of their increasing energies. In other words, electrons first occupy the lowest-energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled.
The order in which the energies of the orbitals increase and hence the order in which the orbitals are filled is as follows:
Is, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d,7p………………..
(ii) Pauli Exclusion Principle: An orbital can have maximum of two electrons and these must have opposite signs.
For example: Two electrons in an orbital can be represented by
The two electrons have opposite spin, if one is revolving clockwise, the other is revolving anticlockwise or vice versa.
(iii) Hund’s Rule of Maximum Multiplicity: Electron pairing in p, d and/orbitals cannot occur until each orbital of a given subshell contains one electron each or is single occupied.
For example: For the element nitrogen which contains 7 electrons, the following configuration can be written.
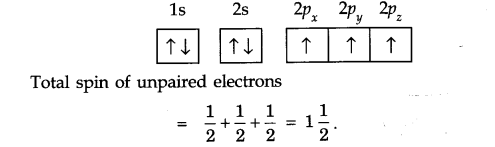
(i) Aufbau principle
(ii) Pauli exclusion principle.
(iii) Hund’s rule of maximum multiplicity.
Answer: (i) Aufbau Principle: In the ground state of the atoms, the orbitals are filled in the order of their increasing energies. In other words, electrons first occupy the lowest-energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled.
The order in which the energies of the orbitals increase and hence the order in which the orbitals are filled is as follows:
Is, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d,7p………………..
(ii) Pauli Exclusion Principle: An orbital can have maximum of two electrons and these must have opposite signs.
For example: Two electrons in an orbital can be represented by
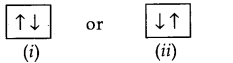
The two electrons have opposite spin, if one is revolving clockwise, the other is revolving anticlockwise or vice versa.
(iii) Hund’s Rule of Maximum Multiplicity: Electron pairing in p, d and/orbitals cannot occur until each orbital of a given subshell contains one electron each or is single occupied.
For example: For the element nitrogen which contains 7 electrons, the following configuration can be written.

IV. Multiple Choice Questions
Question 1. Cathode rays are deflected by
(a) electric field only (b) electric and magnetic field
(c) magnetic field only (d) none of these
Question 2. In a sodium atom (atomic number = 11 and mass number = 23) and the number of neutrons is
(a) equal to the number of protons
(b) less than the number of protons
(c) greater than the number of protons
(d) none of these
Question 3. The Balmer series in the spectrum of hydrogen atom falls in
(a) ultraviolet region (b) visible region
(c) infrared region (d) none of these
Question 4. The idea of stationary orbits was first given by
(a) Rutherford (b) J.J. Thomson (c) Niels Bohr (d) Max Planck
Question 5. de Broglie equation is
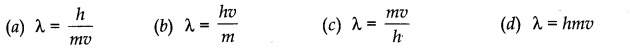
Question 6. The orbital with n = 3 and l = 2 is ,
(a) 3s (b) 3p (c) 3d (d) 3j
Question 7. The outermost electronic configuration of manganese (at. no. = 25) is
(a) 3d5 4s2 (b) 3d6 4s1 (c) 3d74s° (d) 3d6 4s2
Question 8. The energy needed to remove a single electron (most loosely bound) from an isolated – gaseous atom is called
(a) ionisation energy (b) electronegativity
(c) kinetic energy (d) electron affinity
Question 9. The maximum number of electrons in a sub-shell is given by the equation
(a) n2 (b) 2n2 (c) 2l – 1 (d) 2l + 1
Question 10. If the value of azimuthal quantum number is 2, what will be the values for magnetic quantum number?
(a) 2 (b) 3 (c) 4 (d) 5
Answer: 1. (b) 2. (c) 3. (b) 4. (c) 5. (a)
6. (c) 7. (a) 8. (a) 9. (d) 10. (d)
Question 1. Cathode rays are deflected by
(a) electric field only (b) electric and magnetic field
(c) magnetic field only (d) none of these
Question 2. In a sodium atom (atomic number = 11 and mass number = 23) and the number of neutrons is
(a) equal to the number of protons
(b) less than the number of protons
(c) greater than the number of protons
(d) none of these
Question 3. The Balmer series in the spectrum of hydrogen atom falls in
(a) ultraviolet region (b) visible region
(c) infrared region (d) none of these
Question 4. The idea of stationary orbits was first given by
(a) Rutherford (b) J.J. Thomson (c) Niels Bohr (d) Max Planck
Question 5. de Broglie equation is
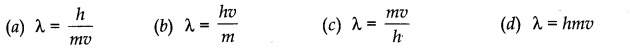
Question 6. The orbital with n = 3 and l = 2 is ,
(a) 3s (b) 3p (c) 3d (d) 3j
Question 7. The outermost electronic configuration of manganese (at. no. = 25) is
(a) 3d5 4s2 (b) 3d6 4s1 (c) 3d74s° (d) 3d6 4s2
Question 8. The energy needed to remove a single electron (most loosely bound) from an isolated – gaseous atom is called
(a) ionisation energy (b) electronegativity
(c) kinetic energy (d) electron affinity
Question 9. The maximum number of electrons in a sub-shell is given by the equation
(a) n2 (b) 2n2 (c) 2l – 1 (d) 2l + 1
Question 10. If the value of azimuthal quantum number is 2, what will be the values for magnetic quantum number?
(a) 2 (b) 3 (c) 4 (d) 5
Answer: 1. (b) 2. (c) 3. (b) 4. (c) 5. (a)
6. (c) 7. (a) 8. (a) 9. (d) 10. (d)
V. Hots Questions
Question 1. Giue flic name and atomic number of the inert gas atom in which the total number of d-electrons is equal to the difference between the numbers of total p and total s-electrons.
Answer: Electronic configuration of Kr (atomic no. = 36) =1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 Total no. of s-electrons = 8 Total no. of p-electrons = 18 Difference = 10, no. of d-electrons = 10
Question 1. Giue flic name and atomic number of the inert gas atom in which the total number of d-electrons is equal to the difference between the numbers of total p and total s-electrons.
Answer: Electronic configuration of Kr (atomic no. = 36) =1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 Total no. of s-electrons = 8 Total no. of p-electrons = 18 Difference = 10, no. of d-electrons = 10
Question 2. What is the minimum product of uncertainty in position and momentum of an electron?
Answer: h/4π
Answer: h/4π
Question 3. Which orbital is non-directional?
Answer: s-orbital.
Answer: s-orbital.
Question 4. What is the difference between the notations l and L?
Answer: I represents the sub shell and L represents shell.
Answer: I represents the sub shell and L represents shell.
Question 5. How many electrons in an atom can have n + l = 6?
Answer: 18.
Answer: 18.
Question 6. An anion A3+has 18 electrons. Write the atomic number of A.
Answer: 15.
Answer: 15.
Question 7. Arrange the electron (e), protons (p) and alpha particle (α) in the increasing order for the values of e/m (charge/mass).
Answer: α < p < e.
Answer: α < p < e.
